Graphene has had a lot of press coverage in recent times and is often dubbed a “super material”.
So much so in fact that researchers from all over the world have been falling over themselves to get a better understanding of it.
So if you’ve ever wondered what it is and what all the hype is about, then you’ve come to the right place.
What You Will Learn
This is an ultimate guide to Graphene but for non-scientists, i.e. for people like you and me.
So I will try to make it as informative and as interesting as possible. So here’s what you’ll learn:
- The story of Graphene
- What Graphene can do
- Applications of Graphene
- The future of Graphene
Even if you’ve been living under a rock for the past decade, you’ve probably heard of Graphene.
If you haven’t, you’ve actually made some. Simply by drawing with a pencil.
What is Graphene?
So what is Graphene? Well in a nutshell it is millions of ultra-thin layers that when stacked together, form graphite, a material that we are all familiar with – its commonly found in pencils.
Graphene is the world’s thinnest material and has the potential to revolutionize almost every part of our daily lives.
It has some awesome physical properties too and has a lot of potential applications.
The most remarkable thing about Graphene? It’s just one atom thick!
However, even though scientists have long since known about one atom thick, two-dimensional crystal graphene, it was only in 2004 that researchers had worked out a way of extracting it from graphite.
A little bit of history
Graphene was first studied in 1947 and in 1984, it was discovered that, in theory, electric current could be carried by effectively mass-less charge carriers in Graphene.
The name ‘Graphene’ first came about in 1987 when the word was used to describe graphite layers that had various compounds inserted between them. Researchers in carbon nanotube technology, which are effectively rolled up sheets of graphene, used the word extensively.
Researchers have been trying to grow graphene on other other single crystal surfaces since the 1970s, but this has proven difficult due to strong interactions with the surface on which graphene was grown.
As such this has always prevented graphene from showing its true potential.
The story of Graphene
The discovery of Graphene was actually a brilliant piece of serendipity. In fact it was so brilliant that it’s on par with the discovery of penicillin by Alexander Fleming. It may even prove just as valuable!
In 2004 two researchers Prof Andre Geim and Prof Kostya Novoselov from the University of Manchester in the UK made an accidental discovery and managed to isolate Graphene for the first time.
So what do I mean by accidental discovery? Well outside of their regular research, Andre and Kostya would hold regular experiments on Fridays.
Andre and Kostya saw these experiments as both a useful way of maintaining interest and a way of generating new ideas.
In their experiments, the two scientists were playing about with flakes of carbon graphite to investigate its electrical properties but ended up trying to see if they could make thinner flakes with sticky Scotch tape!
The scientists used the tape to peel off a layer of graphite from its block. They then repeatedly peeled off further layers from the original flake until they got down to flakes just a few atoms thick.
The researchers had isolated graphene for the first time and created a material known for its unique and immensely interesting properties.
Once the scientists had managed to isolate graphene, they began testing the mono layer material under a microscope.
They published their findings in a paper in 2003 and was rejected twice since it was thought that such single atom sheets would be unstable.
The paper was submitted again in 2004 to the journal Science and was accepted and as a result, it sparked global interest in graphene research.
The interest in material was so great that research groups from around the world sent students to The University of Manchester to learn how to make the material and explore its unusual electronic properties.
The 2010 Nobel Prize in Physics
The discovery of Graphene was so great that the pair won the Nobel prize in Physics in 2010 for their ground-breaking experiments.
The Nobel committee even noted the scientists “playfulness” that aided this pioneering discovery.
Although playing around on Fridays sounds like a jokey thing to do, the scientists turned it into something serious. And if it wasn’t for the remarkable minds of the scientists, it may well have remained a joke and not a serious scientific discovery.
The scientists are ultra committed too. On the same day they received the Nobel prize, they were back in their lab trying to find new and exciting properties of Graphene and other similar two-dimensional crystal materials.
What can Graphene do?
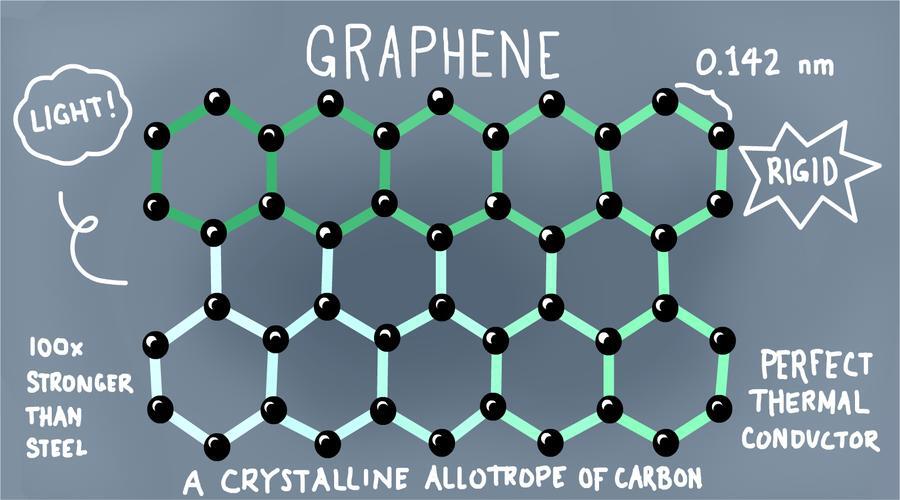
Graphene is a two-dimensional crystal of pure carbon, and its the thinnest and strongest substance known to science. Let’s look a some remarkable facts about this wonder material.
Graphene is super thin
Graphene is a one-atom thin layer of mineral graphite and about one million times thinner than a human hair. Despite being only one atom thick, it’s still visible when you put it next to a white piece of paper.
Graphene is two-dimensional
Mathematically it’s two dimensional and made of carbon, and if you were to zoom in to the molecular level, its hexagon lattice structure would look just like chicken wire!
Graphene is expensive
Graphene is currently quite expensive to manufacture even though it is derived from pencil lead . Enough graphene to cover the head of a pin would cost upwards of $1000!
Work is on the way though to reduce its cost so that it can be used to make transistors and other bits of electronics that are in computing devices which we use every day.
Graphene is flexible
Graphene is incredibly flexible and can be stretched by 20 percent. It’s also remarkably hard, in fact, it’s harder than diamond!
Graphene is Silicon 2.0
It’s been dubbed Silicon 2.0 because it’s an excellent conductor of heat and electricity like silicon. It’s intrinsic mobility is actually a hundred times greater than that of silicon, which means electrons move through graphene with much greater ease and has pretty much no resistance.
Because it is so good at conducting electricity, it may be possible to make batteries that have ten times as much electrical retention capacity as anything else we’ve got.
Graphene is an excellent conductor of heat
Graphene is also an excellent conductor of heat, and beats diamond in this department too! Weirdly, graphene shrinks when it is warmed up and expands when it is cooled down, quite the opposite of every other material on this planet.
Because of this remarkable property, it is the only known example of what scientists call an electrically conductive membrane.
Graphene is ultra strong
Graphene is actually 200 times stronger than steel, in fact a sheet of it as thin as clingfilm, could support an elephant!
Applications of Graphene
Graphene is a disruptive technology which could open many doors to new markets, and could replace existing technologies and materials.
The potential uses for Graphene are potentially limitless. Uses range from new types of flexible electronics that could be worn on clothes or folded up into your pocket.
Graphene could even bring in a new generation of ultra small computers, hyper-efficient solar panels and super-fast mobile phones. But let’s look at some more specific examples:
Drinking water
Graphene has the power to desalinate seawater and make it drinkable. Scientists think that passing seawater through Graphene’s tiny pores in its crystal lattice would let water molecules through but block out salt atoms.
In fact Lockheed Martin have already achieved it with an atom-thick graphene filter.

Super fast charging batteries
Today’s rechargeable batteries are pretty annoying. They take a while to charge up and over time they lose their charging capacity.
However, researchers at UCLA have now found a way to make graphene batteries that charge super fast, are none toxic and inexpensive to produce.
For example an iPhone powered by a graphene supercapacitor could charge in just 5 seconds and a MacBook could charge in just 30 seconds and electric cars could be charged as quickly as it takes us to fill a car with a tank of gas.

Unbreakable smartphone screens
Touchscreens could use graphene as their conductor and could be put onto plastic rather than glass. This would create screens which are light, flexible and unbreakable so you will never have to worry about shattering your phone ever again. Smartphones will also be as thin as a piece of paper but foldable too so that they could fit in your pocket.

Image credit: Flickr
Chinese company Moxi have already developed a phone with a flexible screen. The first devices it will ship have black and white screens, with full colour versions coming soon.
The handset will have a 5.2 inch flexible screen, will weigh 200g and are designed to be rolled into a bracelet and worn on the wrist.
Computers
The original use case for Graphene was to see if it could be used as a transistor – the fundamental electronic switch used in all electronic devices.
It is hoped one day that graphene can replace silicon chips, at least that’s what researchers at MIT hope. They have used Graphene to make chips which are a million times faster than chips we have today.
The future of Graphene
Graphene has a very important future and will ultimately change the world.
It could provide clean drinking water for millions in developing countries and provide more efficient desalination plants.
Electronics could be also be revolutionized with flexible, durable and semi-transparent phones and wearable electronics.
Graphene also has a great potential for use in solar cells. Typical solar cells use silicon to produce charge when a photon hits the material.
Silicon only releases one electron per photon that hits it but with graphene, multiple electrons could be released per photon.
With all these extraordinary claims and a seemingly endless list of strengths, why has graphene not been widely adopted?
Well as usual, it comes down to money. It’s still very expensive to produce in large quantities.
But don’t worry, graphene research is definitely not slowing down. Research laboratories all over the world including the University of Manchester, where graphene was first discovered are continually filing patents for new methods of creating and using graphene.
But revolutions don’t happen over night. Silicon was discovered in the mid 19th century, and it took nearly a century before silicon semiconductors made it into mainstream computing.
So given enough money and time, I definitely see Graphene powering the next era of computing. What do you think? Let me know in the comments below.

